Prune and Plum Fertilization Guidelines
Funding provided by:

- Young Trees
- Bloom
Spring - Fruit Development
Summer - Post-Harvest
Fall - Dormancy
Winter
Nitrogen
(N) 
Prune and Plum Nitrogen Nutrition
Deficiency Symptoms
Nitrogen deficiency results in smaller, pale leaves and reduced shoot growth. Yields can be reduced by decreased flower numbers and shoot growth. Nitrogen deficient trees are also more susceptible to bacterial canker [N27,N32]. With severe deficiencies, leaves may have a scorched appearance and trees may defoliate early [N16]. Nitrogen deficiency is difficult to diagnose from the symptoms alone, and is important to confirm with tissue analyses [N25].
Excessive Nitrogen
Too much N causes excessive shoot growth in both plums and prunes, which increases pruning costs and shades the developing fruit in the lower canopy, delaying maturity and decreasing sugar content. Color and fruit quality of plums and prunes may also be diminished [N7,N16]. Excessively N-fertilized trees are also more at risk of brown rot [N16,N25].
Phosphorus
(P2O5) 
Prune and Plum Phosphorus Nutrition
Deficiency Symptoms
Phosphorus deficiency is very rare in California prune and plum orchards. Stone fruits demand little P, as little is removed with harvested fruits and P is efficiently recycled from the leaves before they drop [P13].
Leaves of P deficient stone fruits are smaller than normal, dark green, have a leathery texture, and may drop early. A purple or reddish color develops on the leaves, stems and young shoots. Symptoms are most marked while shoots are actively growing, and decrease or disappear when vegetative growth slows [P11]. Fruits are smaller, ripen earlier and have a poor flavor. Overall yields are reduced [P13].
A photo of severe P deficiency in Japanese plum can be be found here.
Potassium
(K2O) 
Prune and Plum Potassium Nutrition
Deficiency Symptoms
Prunes are heavy K feeders, and require more K than any other nutrient. Potassium deficiency reduces prune size and soluble solids and increases drying ratio [K16,K21,K22]. Deficiencies are strongly dependent on fruit load and may develop quickly while the tree is filling a heavy crop [K19]. Deficiency symptoms are most pronounced in the most exposed parts of the tree, in hot, dry weather, and under heavy crops [K14,K19].
Symptoms of K deficiency generally appear in midsummer, and are most noticeable in the upper canopy. Leaves first appear pale, and may develop a tan color with scorching along the margins [K15]. Under hot temperatures or with heavy cropping whole leaves may become scorched and drop, making the tree susceptible to bark sunburn. This creates vulnerability to cytospora canker infection, which can lead to limb and scaffold death [K20]. Limb "dieback" through the top of the tree is characteristic of severe deficiencies [K6,K16,K19,K21]. After harvest, the upper branches and shoots of K deficient trees are often bare, having been defoliated by the shaker [K24]. Serious K deficiencies may lead to yield reductions for many years, and if untreated may kill the tree [K19].
Potassium deficiency is a less serious problem on Japanese plums than on prunes, and Japanese plums are also somewhat resistant to cytospora infection [K8,K18,K26].
Zinc
(Zn) 
Prune and Plum Zinc Nutrition
Deficiency Symptoms
Zinc deficiencies are common in plum and prune orchards throughout California, especially on sandy soils and in orchards established on former corrals or barnyards. Zinc availability is also limited on soils with pH greater than 6.5, or that have been heavily manured [Zn2,Zn7,Zn11,Zn13]. Leaf analyses done on prune orchards around the Sacramento Valley in 2003-2004 as part of the Integrated Prune Fertility Project identified Zn deficiency in 72-84% of the orchards sampled [Zn10].
Symptoms of Zn deficiency are most obvious in the spring, particularly if the deficiency is severe. Flower and leaf bud opening is delayed. When they do open, leaves are small, yellowish and form rosettes or tufts, the condition known as "little leaf" [Zn13]. Under slight deficiencies, leaf size is only slightly reduced [Zn13]. Affected leaves may have wavy margins, and dormant flower buds may drop [Zn3]. Dieback may occur under severe deficiencies [Zn8]. Fruits on Zn-deficient shoots are smaller than normal, and in severe deficiencies the entire yield may be lost [Zn7,Zn13].
Soil Test 
Soil Analysis
Soil nitrate analyses are less common in orchards than in annual crops. However, soil nitrate analyses can provide information for the fine-tuning of the fertilization program. This may be especially valuable in drought years, when soil nitrate from the previous season is not leached.
Soil nitrate-N present in spring contributes to the tree’s N nutrition. One ppm of NO3-N per foot of soil corresponds to 3.5-4 lbs N/acre. For example, a NO3-N concentration of 5 ppm in the top foot of the profile corresponds to 17.5-20 lbs N/acre. The N application rate should be adjusted for high residual nitrate concentrations.
Leaf analysis 
Leaf Analysis
In orchards, leaf analyses are more reliable for the diagnosis of nutrient deficiencies than soil analyses. Leaf analyses allow growers to evaluate their fertilization program
Sampling Procedure 
Leaf N should be monitored yearly, as N is very mobile in soil and may change quickly in soil systems [N25]. Leaf nutrient concentrations drop sharply from spring through spring and into summer, and stabilize around July. For this reason, University of California critical nutrient values have been established for July sampling, at which point nutrient concentrations are relatively stable. Samples taken earlier do not currently have any reliable interpretation [N26].
Leaf sampling should be done as follows [N13,N25,N33]:
- The orchard should be divided into uniform blocks with respect to soil type, tree age, variety, location and management.
- Unhealthy, stunted or injured trees are avoided and non-representative areas are sampled separately.
- Fully expanded leaves from non-bearing spurs 5-7 feet above the ground are chosen.
- One or two normal, healthy leaves are collected from each tree for a total of 50-75 leaves per sample.
- Visual evaluation of orchard health and vigor should be made and recorded. This will aid interpretation of test results.
- Samples are submitted to a lab as soon as possible. If a delay is anticipated, they should be refrigerated until they can be sent.
Interpretation of Results 
The critical values presented in the table are conservative; they represent the minimum concentrations necessary for optimal growth [N25]. Leaf concentrations greater than 2.8-3.0% indicate over-fertilization. Visual observations of tree vigor, crop load and leaf color may also be used to interpret test results.
If the test shows adequate leaf N, no N is needed
until mid-April of the following year, at which
point the rate may be decided based on the crop
load. If the test N is low or marginal, a soil N
application several weeks after petal fall may be
needed, regardless of crop load [N24].
To correct an N deficiency mid-season, foliar urea
sprays can be used (see Foliar N). An
in-season soil application may also be used, but
should be very light since stone fruit trees take up
little N in fall and excess N may be leached with
winter rains [N25].
Leaf critical nutrient values from nonbearing spur
leaves of French prune sampled in July [N33].
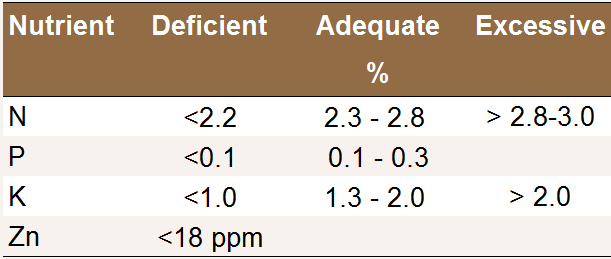
The values in the table refer to a tree. In contrast, the results from leaf analyses refer to the average of all trees sampled in the orchard with some trees being below and others above the average. To minimize the risk of some trees in the orchard being deficient, the lower limit of the adequate range may be increased to 2.4-2.5% N may be more appropriate (Niederholzer, personal communication).
The critical values for N and P are also valid for Japanese plums [N1]. If prunes are produced for the fresh market the lower end of the optimal N range should be used, because of the importance of early maturity [N8].
Soil Test
Soil Analysis
Soil nitrate analyses are less common in orchards than in annual crops. However, soil nitrate analyses can provide information for the fine-tuning of the fertilization program.
High soil nitrate concentrations in late fall may be the result of excess N fertilizer applications during the growing season. Nitrate in the soil profile in fall is prone to leaching during the winter. High nitrate N concentrations below the root zone indicate that in addition to N, irrigation rates were in excess of plant demand.
N 
N Fertilization of Young Trees
Application Rate 
Nitrogen is important to biomass growth, and young
trees benefit from N fertilizer [N31];
however, immature trees require less N that mature
trees as they are smaller and produce a smaller crop
[N25]. In an
orchard in Yolo County on a clay loam soil,
Southwick and coworkers [N31]
found that trees fertigated with an annual total of
0.25-0.5 lbs N/tree regularly during the season had
good growth and high yields, while the incidence of
bacterial canker was minimized. Based on these
results and taking into account the increasing N
requirements as trees grow, Niederholzer and
coauthors [N25]
recommended the application rates for young trees
summarized in the table. These application rates are
in line with earlier recommendations by La Rue and
Gerdts [N16].
Optimal N application rates to young prune trees
planted at a spacing of 14 x 17 feet (183
trees/acre) [N25].
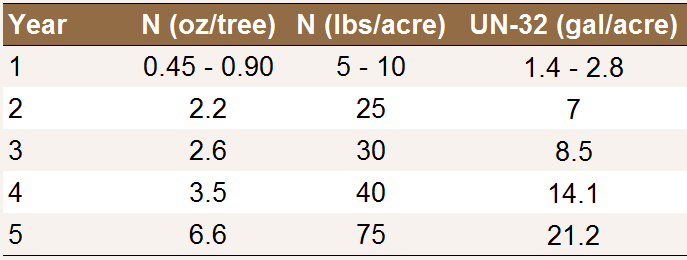
In loam, clay loam or sandy clay loam soils, the soil may provide sufficient N to support the first year of growth [N15,N32]. In order to protect against fertilizer burn, rates should be reduced if applications are done in hot weather when trees are taking up more water [N9]. This is especially true where liquid fertilizers are used, as they can easily burn young trees [N11].
Fertilizer Type 
Experience with almonds suggests that liquid fertilizers more easily burn young roots and may be dangerous to use with first-leaf trees [N11]. A conservative approach is to use a granular fertilizer, placed at least 18 inches from the trunk but within the wetting zone of the irrigation system [N9]. Blends such as 12-12-12 and 15-15-15 also supply P and K. For fertigation, UN32 is commonly used [N25].
Foliar N 
Foliar Nitrogen
Foliar N applications have not been found to increase yields or fruit size in non-deficient orchards [N29,N30]. However, they may be useful for addressing in-season deficiencies or for applications under conditions where soil uptake is likely to be low, such as when soil is very cold or wet in spring, or following harvest [N18,N20,N36]. Research on deciduous fruit trees in general suggests that foliar N cannot replace soil-applied N, and that a response may only be expected if tree demand is higher than the amount the soil can supply [N36].
Application Rate and Time 
Rates must be kept low to avoid leaf burn and the multiple applications needed may become expensive [N17,N34]. Because of this, foliar N can supplement but not replace soil applications. Spring urea sprays of 1.8% or higher have been shown to damage leaves of stone fruits, and sprays of potassium nitrate exceeding 1.2% have damaged prune leaves [N34]. Higher rates, up to 4 to 5% of low-biuret urea, have been safely applied post-harvest [N17].
Soil applied N 
Soil Applied N
Application Rate 
About 10-15 lbs of N are removed per dry ton of
prunes [N5,N24,N31,N37].
For more information see Nitrate Uptake and Partitioning.
In addition, based on values from other tree species
it is estimated that about 30-40 lbs N/acre/year are
required for vegetative growth in a mature orchard [N23]. Because not
all N applied is taken up by the tree, total N input
from all sources needs to be higher than this. The
values in the table assume N application through
drip or microsprinkers and an N use efficiency of
70%.
Annual N application rates to prunes fertigated via
low volume irrigation. N input includes fertilizer N
as well as N in the irrigation water.
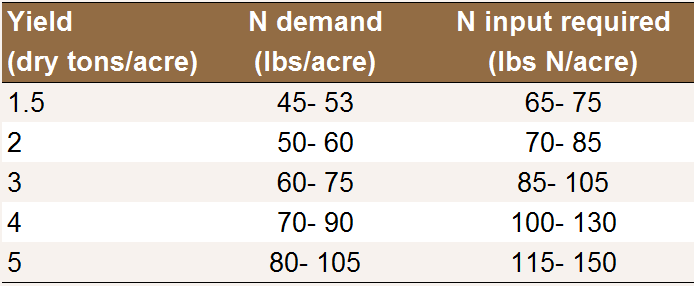
The values in the table are in line with Johnson and Uriu [N12], who found that 100-150 lbs N/acre are usually adequate for plums, peaches and nectarines.
Similar rates are recommended for mature plums and prunes grown for fresh market. Early season varieties should be fertilized less than those harvested later in the season [N8,N12]. Leaf analyses provide a means to evaluate the N fertilization program.
Nitrate in the Irrigation Water 
When well water is used for irrigation, it may contribute a significant amount of N [N28]. Well water samples should be taken after the pump has been run for several hours, to make sure that the water sampled is representative of what the orchard will receive [N23]. Nitrate in well water can be calculated as the ppm of nitrate-N x 2.72 = lbs N applied in one acre foot of water.
For example, a test value of 7 ppm nitrate-N in an orchard receiving 2 acre-feet of water per year would mean that 38 lbs N/acre are added annually with the irrigation water. If the concentration of nitrate rather than nitrate-N is given, multiply by 0.614 rather than 2.72. The proportion of irrigation water N available to the trees depends on how carefully irrigation water is managed [N5]. To estimate N credits, only the nitrate-N in the water taken up by the trees, which corresponds to the evapotranspiration (ET) rate, should be counted [N23].
Mode of Application 
Application through low-volume irrigation systems is more efficient than broadcasting or banding [N14].
When applied through fertigation, the fertilizer should be injected into the irrigation system in the middle third of the irrigation set. For example, in an 18-hour irrigation set, fertilizer is injected from hour 6 through hour 12. This prevents urea and nitrate from moving below the root zone but still ensures that the N is distributed well in the wetting zone and not overly concentrated, causing root burn [N23].
In orchards where N is not fertigated, the fertilizers should be applied in the herbicide strip along the tree rows and not broadcast over the entire area. Fertilizer applied between the rows is less efficiently used due to competition with weeds or cover crops and lower prune root density compared to the tree row [N22]. Surface applied N should be incorporated or irrigated into the root zone shortly after application [N21]. This is especially important when fertilizers containing ammonium or urea are used in hot, windy weather and on soils with an alkaline pH because these factors increase ammonia volatilization.
Fertilizer Type 
A number of mineral N fertilizers are available to growers. Mineral fertilizers contain N in the form of urea, ammonium, nitrate or a mix of them. Research with almonds suggests that when losses are minimized and equal amounts of N are added, the effects on yield of these fertilizers are similar, and this is likely true for plums and prunes as well [N3,N4,N23,N25]. The N forms, however, behave differently in the environment. Nitrate is very mobile in the soil and can easily be leached into deeper soil layers with irrigation water or rain. Ammonium is less mobile, but is generally converted quickly to nitrate by soil microorganisms, especially in warm and moist soils, unless they are water saturated. This process, nitrification, can lower soil pH. The acidifying effect is especially strong when ammonium fertilizers are applied by drip systems as they are concentrated in a small soil volume [N37]. Ammonium may volatilize as ammonia, especially when applied to the surface of dry and alkaline soils without incorporation. Urea is relatively mobile in the soil, but is generally quickly converted to ammonium.
More detailed information about the most common fertilizers can be found on the website of the IPNI.
Time of Application 
Very little soil N is taken up during dormancy and before bud swelling. While N is needed for flowering it is recycled from the perennial parts of the tree rather than taken up from the soil for immediate use [N35]. The majority of N uptake occurs during the period of rapid shoot growth, with about 60-70% of the total N requirement being taken up by June [N6]. The N taken up during the late summer supports flowering the following spring [N35]. Uptake slows during fall through leaf drop, and is essentially zero through winter [N22]. For this reason N fertilizers should not be applied after leaf drop, in winter or in spring before bud break as they have a high risk of being leached out before the crop can use them.
Based on the seasonal uptake pattern, the first N application in spring is best done around mid-April, before rapid uptake is beginning. Exceptions are marginal or N-deficient orchards where some N should be applied a few weeks after petal fall [N24].
Multiple small applications are more efficient than large applications; Niederholzer [N23] recommends not more than 20-30% of the annual fertilizer budget should be added per application. A general rule in a healthy orchard with adequate tree vigor is that one-third of the annual N requirement may be applied in spring (March or April), with the remaining two thirds applied in mid-to late summer (June, July and August). Where tree vigor is low, a relatively greater proportion should be applied in spring, and the remainder applied in summer [N22]. Applications after harvest should be small and applied well before leaf fall, so that the N can be taken up and stored in the tree's woody parts [N21,N25].
Soil Test 
Soil Analysis
Soil analyses have not been shown to be a reliable means of determining prune response to fertilizers, and the University of California has not developed critical values for soil analysis [P9]. However, soil analyses may be useful to monitor trends in nutrient availability over the years.
Soil analyses are also useful prior to orchard establishment to determine pH and identify potential problems, i.e. salinity, the presence of toxic ions such as boron or chloride, and the presence of limiting layers [P9].
Soil Sampling and Analysis
Soil samples for nutrient analysis should be taken from the main root zone. Most roots of plum trees are in the top 2 feet of the profile [P15]. When the soil samples are also used to assess soil salinity, sampling to a depth of 4 feet may be required [P8]. For more information on sampling procedure see Soil Sampling in Orchards.
Phosphorus availability strongly depends on soil pH. In acidic soils, P is immobilized by iron and aluminum minerals, while the predominant mechanism of P immobilization in alkaline soils is the formation of insoluble calcium phosphate compounds. For this reason, the recommended soil test depends on soil pH. For acidic to neutral soil (pH < 7.0), the Bray P1 test is generally used. For neutral and alkaline soils (pH > 6.5), the Olsen method is more appropriate [P7]. Using a test that is inappropriate for the soil analyzed may result in inaccurate results.
Interpretation of Test Results 
The University of California has not developed
critical values for soil analysis in prune or plum
orchards [P9].
The table below gives some general guidelines for
soil test interpretation in orchards.
Interpretation of P and K soil test results in
orchards [P7].
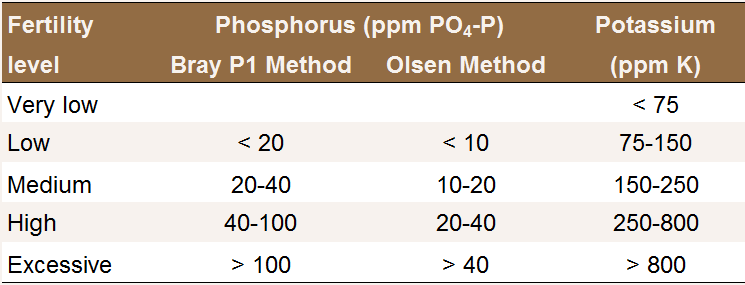
When soil or leaf analyses suggest that P fertilization may be beneficial, the application rate can be based on the amount of P removed with harvested fruits each year (see P Fertilization of Mature Trees).
Leaf analysis 
Leaf Analysis
In orchards, leaf analyses are more reliable for the diagnosis of nutrient deficiencies than soil analyses. Leaf analyses allow growers to evaluate their fertilization program.
Sampling Procedure 
Leaf nutrient concentrations drop sharply from spring through spring and into summer, and stabilize around July. For this reason, University of California critical nutrient values have been established for July sampling, at which point nutrient concentrations are relatively stable. Samples taken earlier do not currently have any reliable interpretation [P10].
Leaf sampling should be done as follows [P6,P9,P14]:
- The orchard should be divided into uniform blocks with respect to soil type, tree age, variety, location and management.
- Unhealthy, stunted or injured trees are avoided and non-representative areas are sampled separately.
- Fully expanded leaves from non-bearing spurs 5-7 feet above the ground are chosen.
- One or two normal, healthy leaves are collected from each tree for a total of 50-75 leaves per sample.
- Visual evaluation of orchard health and vigor should be made and recorded. This will aid interpretation of test results.
- Samples are submitted to a lab as soon as possible. If a delay is anticipated, they should be refrigerated until they can be sent.
Interpretation of Results 
Leaf critical nutrient values from nonbearing spur
leaves of French prune sampled in July [P14].

The values in the table refer to a tree. In contrast, the results from leaf analyses refer to the average of all trees sampled in the orchard with some trees being below and others above the average. To minimize the risk of some trees in the orchard being deficient, the lower limit of the adequate range may be increased to 0.15% P (Niederholzer, personal communication).
Leaves of Japanese plum have similar critical values for N and P [P1].
Soil Test 
Soil Analysis
Soil analyses have not been shown to be a reliable means of determining prune response to fertilizers, and the University of California has not developed critical values for soil analysis [P9]. However, soil analyses may be useful to monitor trends in nutrient availability over the years.
Soil analyses are also useful prior to orchard establishment to determine pH and identify potential problems, i.e. salinity, the presence of toxic ions such as boron or chloride, and the presence of limiting layers [P9].
Soil Sampling and Analysis
Soil samples for nutrient analysis should be taken from the main root zone. Most roots of plum trees are in the top 2 feet of the profile [P15]. When the soil samples are also used to assess soil salinity, sampling to a depth of 4 feet may be required [P8]. For more information on sampling procedure see Soil Sampling in Orchards.
Phosphorus availability strongly depends on soil pH. In acidic soils, P is immobilized by iron and aluminum minerals, while the predominant mechanism of P immobilization in alkaline soils is the formation of insoluble calcium phosphate compounds. For this reason, the recommended soil test depends on soil pH. For acidic to neutral soil (pH < 7.0), the Bray P1 test is generally used. For neutral and alkaline soils (pH > 6.5), the Olsen method is more appropriate [P7]. Using a test that is inappropriate for the soil analyzed may result in inaccurate results.
Interpretation of Test Results 
The University of California has not developed
critical values for soil analysis in prune or plum
orchards [P9].
The table below gives some general guidelines for
soil test interpretation in orchards.
Interpretation of P and K soil test results in
orchards [P7].

When soil or leaf analyses suggest that P fertilization may be beneficial, the application rate can be based on the amount of P removed with harvested fruits each year (see P Fertilization of Mature Trees).
P 
P Fertilization of Young Trees
If an orchard is planted on soils known to be P-deficient, triple-superphosphate may be added to the planting hole, making sure that it will not be in direct contact with the tree roots [P13].
Little research has been done on the P needs of young prune or plum trees. In almonds, which are closely related, small applications of a low-analysis granular fertilizer such as 15-15-15 and 12-12-12 have been found to be useful in providing adequate P and K for the period of rapid growth following the first year [P4].
Soil applied P 
Soil Applied Phosphorus
Phosphorus deficiency has rarely been observed in stone fruit orchards in California [P5]. There are currently no University of California recommendations for P fertilization of prune and plum orchards. Soil and leaf analyses can be used to monitor the P status of an orchard over the years to ensure that the P availability is adequate (see Soil P Test and Leaf P Analysis).
Application Rate 
In the long term, the amount of P removed at harvest needs to be replaced with fertilizer to maintain adequate P availability. Approximately 5 lbs P2O5 (2.2 lbs P) are removed from the orchard per dry ton of prunes [P2,P12]. For stone fruits grown on P deficient soils, a large application of 350-450 lbs P2O5 per acre may be necessary; this should be effective for several years [P1,P13].
Mode of Application 
Strip or ring applications at the drip line are more effective than broadcast applications [P1].
As P is strongly immobilized by soil minerals, it cannot be leached into the root zone with irrigation or rainwater and should be incorporated into the soil for better availability. Phosphorus fertilizers can be drilled 6 to 8 inches deep in one or two bands on opposite sides of the tree row [P1].
Phosphorus can be fertigated; however care must be taken to prevent the formation of calcium phosphates which can plug the emitters [P3].
Fertilizer Type 
Facts sheets about the most common fertilizers can be found on the IPNI homepage.
Time of Application 
Phosphorus is immobile in the soil and is barely leached. The time of application is therefore not crucial as it is for N. It may be applied with K in November after leaf drop begins.
Soil Test 
Soil Analysis
Soil analyses have not been shown to be a reliable means of determining prune response to fertilizers, and the University of California has not developed critical values for soil analysis [K21]. However, soil analyses may be useful to monitor trends in nutrient availability over the years.
Soil analyses are also useful prior to orchard establishment to determine pH and identify potential problems, i.e. salinity, the presence of toxic ions such as boron or chloride, and the presence of limiting layers [K21].
Soil Sampling and Analysis
Soil samples for nutrient analysis should be taken from the main root zone. Most roots of plum trees are in the top 2 feet of the profile [K31]. When the soil samples are also used to assess soil salinity, sampling to a depth of 4 feet may be required [K12]. For more information on sampling procedure see Soil Sampling in Orchards.
Leaf analysis 
Leaf Analysis
In orchards, leaf analyses are more reliable for the diagnosis of nutrient deficiencies than soil analyses. Leaf analyses allow growers to evaluate their fertilization program.
Sampling Procedure 
Leaf nutrient concentrations drop sharply through spring and into summer, and stabilize around July. For this reason, University of California critical nutrient values have been established for July sampling, at which point nutrient concentrations are relatively stable. Samples taken earlier do not currently have any reliable interpretation [K23].
Leaf sampling should be done as follows [K16,K21,K30]:
- The orchard should be divided into uniform blocks with respect to soil type, tree age, variety, location and management.
- Unhealthy, stunted or injured trees are avoided and non-representative areas are sampled separately.
- Fully expanded leaves from non-bearing spurs 5-7 feet above the ground are chosen.
- One or two normal, healthy leaves are collected from each tree for a total of 50-75 leaves per sample.
- Visual evaluation of orchard health and vigor should be made and recorded. This will aid interpretation of test results.
- Samples are submitted to a lab as soon as possible. If a delay is anticipated, they should be refrigerated until they can be sent.
Interpretation of Results 
Leaf levels are used to determine fall application
rates. If levels are low or marginal and a good crop
was harvested, K fertilizer should be applied [K6]. If leaf
levels are greater than 2% there is unlikely to be
any benefit from additional K fertilization in that
season. However, the K removed by the harvested crop
should still be replaced to maintain soil K
availability [K24]
(see Soil Applied K).
Leaf critical nutrient values from nonbearing spur
leaves of French prune sampled in July [K30].

The values in the table refer to a tree. In contrast, the results from leaf analyses refer to the average of all trees sampled in the orchard with some trees being below and others above the average. To minimize the risk of some trees in the orchard being deficient, the lower limit of the adequate range may be increased to 1.5% K (Niederholzer, personal communication).
For Japanese plum, leaf K values of 1.1% are considered adequate [K1].
May Leaf Interpretation 
July leaf analyses are not very useful for in-season adjustment; and no reliable relationship between earlier leaf samples and July values has been found for prune. However, broadly, if leaf K is near deficiency (less than 1.3%) in May, trees will likely be deficient by July. If leaf K concentrations exceed 2.3% in May, trees are not likely to be deficient in July [K23].
Soil Test 
Soil Analysis
Soil analyses have not been shown to be a reliable means of determining prune response to fertilizers, and the University of California has not developed critical values for soil analysis [K21]. However, soil analyses may be useful to monitor trends in nutrient availability over the years.
Soil analyses are also useful prior to orchard establishment to determine pH and identify potential problems, i.e. salinity, the presence of toxic ions such as boron or chloride, and the presence of limiting layers [K21].
Soil Sampling and Analysis
Soil samples for nutrient analysis should be taken from the main root zone. Most roots of plum trees are in the top 2 feet of the profile [K31]. When the soil samples are also used to assess soil salinity, sampling to a depth of 4 feet may be required [K12]. For more information on sampling procedure see Soil Sampling in Orchards.
K 
K Fertilization of Young Trees
Because a large proportion (about 70%) of a prune tree's K content is found in the fruit, K deficiency is rare in young and nonbearing trees [K21,K19,K32]. Little research has been done on the K needs of young prune trees. In almonds, which are closely related, small applications of a low-analysis granular fertilizer such as 15-15-15 and 12- 12-12 have been found to be useful in providing adequate P and K for the period of rapid growth following the first year [K9].
KCl fertilizer should not be used on young prune or plum trees, as they are sensitive to chloride [K24].
.
Soil applied K 
Soil Applied Potassium - In-Season
A heavy crop of prunes must accumulate a lot of K,
meaning that severe deficiencies may develop quickly even
on soils with normal K concentrations [K19,K27]. Ensuring
that sufficient K is available during fruit development
involves good irrigation management, fruit thinning in
heavy crop years and a consistent supply of in-season K.
Although K- deficiency is less of a problem on plums than
on prunes, recommended rates and methods of deficiency
correction are similar [K18].
Common prune K fertilization strategies [K7].
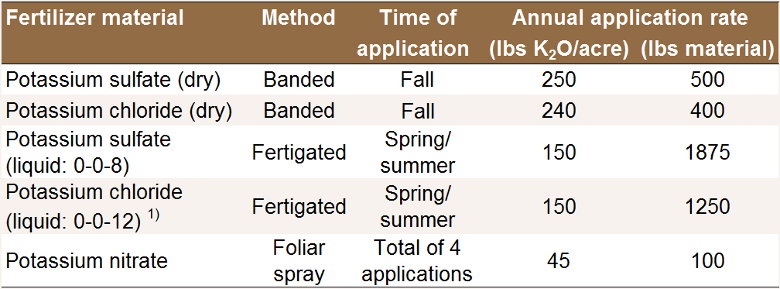
1) Fertigation with KCl can be risky, as
chloride may burn the roots [K7].
Application Rate 
Prunes remove an estimated 23 lbs K2O per dry ton harvested per acre. Assuming that 50% of the fertilizer applied is plant available, about 100 lbs of potassium sulfate fertilizer (0-0-50) per acre would be needed to replace K removed by each ton of yield [K2,K24,K28,K32]. A common recommendation for K applied by fertigation to mature, heavily cropped prune trees is 150 lbs K2O/acre as K2SO4 or KCl [K7]. Since more of the K applied through drip systems is plant available, the application rate can be reduced when fertigation is used compared to when dry fertilizer is banded in fall (240-250 lbs K2O/acre) [K7].
Fruit is the driver of K uptake, so in years where crop load is light (for example, if hot spring weather reduced fruit set) in-season K can be reduced[K7,K24,K32]. If prunes are being grown for the fresh market, the K requirement will be lower because of the lighter crop load [K5].
Soil properties can affect K availability. Higher rates may be required for heavy soils, where more K is held on soil surfaces, compared to lighter soils [K21]. In areas where soils are known to fix K, such as the east side of the San Joaquin Valley, analyzing the soil for its K fixation capacity before the orchard is planted helps adjust the K fertilization program. A discussion about K fixation in the San Joaquin Valley can be found here. For more information about soil K fertilization in your area,contact your local farm advisor specializing in prune or plum production.
Mode of Application 
Broadcast or sprinkler applications do not deliver K directly to the root zone and are less effective than band applications or fertigation through the drip system [K32]. Fertigation is more efficient than banding on fine-textured soils. On a clay loam near Colusa in the Sacramento Valley, Uriu and coworkers [K29] found that mature prune trees receiving 10 lbs drip-applied K2SO4 per tree maintained higher leaf K than trees receiving 20 lbs trenched granular K2SO4 per tree. In addition, when K is repeatedly applied in the same place, K binding sites become saturated and availability is increased [K7,K32].
Since soil-applied K fertilizer may take several months to affect tree K status, dry fertilizers are best applied in fall [K7]. If a heavier crop is set or large prunes are desired, fall fertilization may be supplemented by in-season fertigation or foliar application [K7]. If KCl is used banding the dry fertilizer in fall is the safest method, so the chloride ion can be washed out of root zone over winter, if adequate rainfall occurs. Irrigation water may be needed to leach chloride if insufficient rain falls. See Soil Applied K in Fall for information on applying dry fertilizers.
Fertilizer Type 
Many types of K fertilizer may be used. Potassium sulfate (K2SO4) and potassium chloride (KCl) are the most common [K20].
Although KCl fertigation is possible (see Table), this practice is risky, as chloride may burn the roots [K]. KCl should not be used in orchards with fluctuating water tables or significant drainage obstructions [K20,K23].
Chlorine toxicity on plum and prune appears as a leaf tip burn and marginal scorch [K25]. Where KCl is used, it is a good idea to test July leaf samples for Cl- to monitor status over time. A value of 0.3% Cl- is considered excessive [K16].
Potassium thiosulfate (KTS, 0-0-25) is a liquid fertilizer sometimes used in fertigation. Rates higher than 12 gallons per acre per application may injure or kill trees. Potassium thiosulfate lowers the soil pH [K19].
Time of Application 
When fertigated through the drip system, K is best applied throughout the growing season, starting in late April or early May and going through July [K3,K32]. Prune fruits accumulate K from bloom until harvest [K20]. In-season application methods allow growers to adjust rates if unforeseen events like spring frost or hot weather at blooming lead to a lighter crop than was expected.
Fall applied K 
Soil Applied Potassium - Fall
Granular fertilizers are usually applied in fall. A disadvantage of this approach is that it does not allow rate adjustment based on spring weather and expected crop load.
Application Rate 
Prunes remove an estimated 23 lbs K2O per dry ton harvested per acre. Assuming that 50% of the fertilizer applied is plant available, about 100 lbs of potassium sulfate fertilizer (0-0-50) per acre would be needed to replace K removed by each ton of yield [K2,K24,K28,K32]. A common recommendation for mature, heavily cropped prune trees is 240-250 lbs K2O/acre banded as K2SO4 or KCl. Since more of the K applied through drip systems is plant available, this application rate is higher than when in-season fertigation is used (150 lbs K2O/acre) [K7].
July leaf K analyses and the previous year's crop are used to determine the rate of fall fertilization [K6]. Fruit is the driver of K uptake, so in years where crop load is light less in-season K is necessary, and as long as tissue K levels are acceptable fall K application may be reduced [K7,K24,K32]. In plums or prunes grown for the fresh market, the K requirement will be lower because of the lighter crop load [K5]. If trees are K deficient, higher application rates may be used.
Soil properties can affect K availability. Prunes are not commonly planted on sandy soils, but where they are, lower rates of fall appled K are suggested as the soil has less K holding capacity. Experience with almonds suggests that when soil CEC is less than 15 meq/ 100 g soil, K applications should be reduced [K10]. Conversely on heavier soils higher rates may be required, as more K is held on soil surfaces[K20]. In areas where soils are known to fix K, such as the east side of the San Joaquin Valley, analyzing the soil for its K fixation capacity before the orchard is planted helps adjust the K fertilization program. A discussion about K fixation in the San Joaquin Valley can be found here. For more information about soil K fertilization in your area, contact your local farm advisor specializing in plum or prune production.
Mode of Application 
Broadcast or sprinkler applications do not deliver K directly to the root zone and are less effective than band applications or fertigation through the drip system [K32]. In-season fertigation is more efficient than fall banding on fine-textured soils. On a clay loam near Colusa in the Sacramento Valley, Uriu and coworkers [K28] found that mature prune trees receiving 10 lbs drip-applied K2SO4 per tree maintained higher leaf K than trees receiving 20 lbs trenched granular K2SO4 per tree. In addition, when K is repeatedly applied in the same place, K binding sites become saturated and availability is increased [K7,K31].
Dry fertilizers should be banded or shanked along tree rows, and washed into the soil by rainfall or irrigation water [K6]. On heavier soils, which bind more K, gypsum (CaSO4) is sometimes added in the same band at rates of 1000-4000 lbs per acre [K24]. The calcium ion competes with K for binding sites on soil surfaces, helping it move deeper into the root zone [K4]. Bands should be made in the same place every year [K24].
Fertilizer Type 
Many types of K fertilizer may be used. Potassium sulfate (K2SO4) and potassium chloride (KCl) are the most common [K21]. Because prunes and plums are sensitive to chloride, KCl must be used with care. Usually it is applied in late fall or winter so that winter rains can flush the chloride ion (Cl-) out of the root zone. If there has not been much rain by January, supplementary irrigation to wash it down is advised. KCl should not be used in orchards with fluctuating water tables or significant drainage obstructions [K21,K24].
Foliar K 
Foliar K
A large proportion of the K contained in a mature prune tree is concentrated in the fruits. If there is a heavy crop and large prunes are needed, foliar sprays through July are recommended, especially on soils with marginal K availability [K17]. Research suggests that prunes may receive their entire K requirement foliarly. In a 3-year study in a chronically K-deficient orchard in the north Sacramento Valley, Southwick and coworkers [K27] found no yield, fruit quality or July leaf K differences between trees receiving an annual fall soil application of 600 lbs per acre KCl and trees receiving four annual foliar applications of 25 lbs potassium nitrate (KNO3) per acre over the course of the growing season. A study on Japanese plums in Tunisia found that the trees receiving 100% of their K requirement foliarly maintained similar yields and K status to fertigated trees, and that plums from trees which received at least some foliar K matured earlier, weighed more and had higher total soluble solids, particularly in the earlier harvests [K13].
Application Rate and Time
A total of 100 lbs potassium nitrate (KNO3) per acre in multiple sprays is recommended to avoid K deficiency in heavily cropped trees [K21,K27]. 20-30 lbs KNO3 in 100 gallons of water per acre may be applied at 2 to 3 week intervals, starting in late April to early May, before deficiencies show, and going through July [K7,K16,K27].
No more than 25 lbs KNO3 per 100 gallons per acre should be applied in hot weather, to avoid leaf damage [K21]. If zinc fertilizer is applied in the same spray, no more than 10 lbs KNO3 in 100 gallons water per acre should be used.
Fertilizer Type 
Potassium nitrate is most commonly used. Other foliar K fertilizer are available; however if the entire K demand is being supplied foliarly, annual application rates should be equivalent to a total of 100 lbs KNO3 per acre (45 lbs K2O/acre) [K20].
Soil Test 
Soil Analysis
The Zn status in orchards is most often assessed with leaf analyses. In general, DTPA extractable Zn concentrations of 0.7-1.5 ppm in soil are considered adequate. Concentrations below 0.7 ppm indicate low availability, while concentrations exceeding 1.5 ppm are considered high [Zn1].
Leaf analysis 
Leaf Analysis
In orchards, leaf analyses are more reliable for the diagnosis of nutrient deficiencies than soil analyses. Leaf analyses allow growers to evaluate their fertilization program.
Sampling Procedure 
Leaf nutrient concentrations drop sharply from spring through spring and into summer, and stabilize around July. For this reason, University of California critical nutrient values have been established for July sampling, at which point nutrient concentrations are relatively stable. Samples taken earlier do not currently have any reliable interpretation [Zn10].
Leaf sampling should be done as follows [Zn2,Zn6,Zn8,Zn13]:
- The orchard should be divided into uniform blocks with respect to soil type, tree age, variety, location and management.
- Unhealthy, stunted or injured trees are avoided and non-representative areas are sampled separately.
- Fully expanded leaves from non-bearing spurs 5-7 feet above the ground are chosen.
- Leaves which may have received or been contaminated with Zn from a foliar fertilizer or Zn- containing pesticide spray should not be used.
- One or two normal, healthy leaves are collected from each tree for a total of 50-75 leaves per sample.
- Visual evaluation of orchard health and vigor should be made and recorded. This will aid interpretation of test results. This is particularly important for Zn, for which leaf concentrations may be reduced in a vigorously growing tree.
- Samples are submitted to a lab as soon as possible. If a delay is anticipated, they should be refrigerated until they can be sent.
Interpretation of Results 
If levels are at or around deficiency and a good crop was harvested, Zn fertilizer should be applied, either in fall or in the following year [Zn3].
Leaf Zn levels may be lower in vigorously growing
orchards, and leaf Zn may also be affected by past
foliar fertilizer history. A good practice is to
keep records from past years and track Zn levels
over time. Past experience, and visual observation
of orchard appearance and current growth should be
used alongside leaf analysis to make fertilization
decisions [Zn2].
Leaf critical nutrient values from nonbearing spur
leaves of French prune sampled in July [Zn13].

In a study with Fortune Plums at the Kearney Agricultural Center, Johnson and coworkers [Zn4] found that Zn deficiency symptoms were only present in trees with lead Zn levels below 10 ppm. These results suggest that the deficiency threshold for Zn may be lower than the published threshold [Zn4].
Soil Test 
Soil Analysis
The Zn status in orchards is most often assessed with leaf analyses. In general, DTPA extractable Zn concentrations of 0.7-1.5 ppm in soil are considered adequate. Concentrations below 0.7 ppm indicate low availability, while concentrations exceeding 1.5 ppm are considered high [Zn1].
Zn 
Zn Fertilization of Young Trees
Little research has been done on Zn nutrition of young prune trees. Johnson and Day [Zn5] found that adding the equivalent of 1.4 ounces of Zn per tree to the soil prior to transplanting by mixing a pelletized formulation of Zn oxide and sulfur with soil from the planting hole increased 'Angelus' peach and 'Friar' plum leaf Zn concentrations for several years compared with an unfertilized control. Early experiments suggest that Zn oxide alone would be as effective and less expensive [Zn5]. However, while increasing leaf Zn the technique did not improve tree growth in seedlings that were not Zn deficient.
Foliar Zn 
Foliar Zinc
Zinc is most often applied foliarly to plums and prunes. Foliar Zn application may make symptoms disappear without necessarily increasing leaf Zn concentrations. The application rate must be chosen with care. If too little is applied the leaf may not absorb the fertilizer, and if too much is applied it may become toxic [Zn8].
Zinc application may be done every year or every other year, depending on the site. Application strategies depend on whether Zn is applied in spring, fall, or during dormancy [Zn3,Zn8].
Spring Applications
A variety of Zn fertilizers, including basic zinc sulfate, zinc nitrate, zinc oxide, zinc EDTA and various other zinc chelate products may be used [Zn8].
A general recommendation is for 3-5 lbs of zinc oxide or basic zinc sulfate per 100 gallons of water. This application may be combined with an early-season potassium nitrate application; however, a smaller rate of K fertilizer (10 lbs KNO3 in 100 gallons water per acre) should be used [Zn8]. If spray adjuvants are used the rates may need to be decreased, as they increase uptake and may cause marginal leaf burn [Zn8].
In spring, Zn should be applied preferably before leaves reach their full size and no later than mid- May [Zn9]. Applications for both prune and plum should be avoided if there is rain forecast in the next 48 hours, as leaf rewetting may cause too much Zn uptake, leading to "shot-holing" in the leaves [Zn8].
Foliar Zn 
Foliar Zinc
Zinc is most often applied foliarly to plums and prunes. Foliar Zn application may make symptoms disappear without necessarily increasing leaf Zn concentrations. The application rate must be chosen with care. If too little is applied the leaf may not absorb the fertilizer, and if too much is applied it may become toxic [Zn8].
Zinc application may be done every year or every other year, depending on the site. Application strategies depend on whether Zn is applied in spring, fall, or during dormancy [Zn3,Zn8].
Fall and Dormancy Applications
Normally, only zinc sulfate is applied in fall and during dormancy.
Late Zn applications are done at the beginning of normal leaf drop, or during dormancy. Higher rates may be applied than in spring; from 10-15 lbs zinc sulfate per acre in 100 gallons water is recommended, with a maximum of 20-25 lbs per acre.
Aphid control materials may be sprayed with fall zinc sulfate application, if no oil is included in the mix. Oil should not be applied within 30 days of zinc sulfate [Zn3,Zn8].
Zn applied in fall before leaf drop may accelerate defoliation. If the orchard is being irrigated or rain has recently fallen, Zn sulfate sprays at fall-recommended rates will remove leaves over the course of a week or so (Niederholzer, personal communication). However, this should not affect the following year's buds and may be an advantage in areas where tree blowover or foliar disease overwintering is a concern [Zn3,Zn8]. In dry orchards, when humidity is low and irrigation has stopped, even high Zn sulfate applications don't cause defoliation.
Soil applied Zn 
Soil Applied Zinc
Soil applications of Zn to fruit trees are rarely done, as they are less reliable than foliar applications and require much more fertilizer [Zn12]. Positive effects of soil-applied Zn in fruit trees have been reported with large applications or concentrated fertilizer bands; however, these methods are risky as Zn may easily become toxic [Zn12].
Acknowledgments 
Guidelines and Webpage Design:
- Daniel Geisseler, Ph.D.; UCCE Specialist in Nutrient Management; Department of Land, Air and Water Resources, University of California, Davis
- Patricia Lazicki, Department of Land, Air and Water Resources, University of California, Davis
Reviewers:
- Franz J.A. Niederholzer, Ph.D.; Farm Advisor Orchard Systems; University of California Cooperative Extension Sutter-Yuba Counties
- Katherine Pope, Ph.D.; Farm Advisor Orchard Systems; University of California Cooperative Extension Yolo County
- William R. Horwath, Ph.D.; Professor of Soil Biogeochemistry and James G. Boswell Endowed Chair in Soil Science; Department of Land, Air and Water Resources, University of California, Davis
Support:
- Moradi A. Barzin, Ph.D.; Senior Environmental
Scientist FREP, California Department of Food and
Agriculture
- Amadou Ba, Ph.D.; Branch Chief Feed,
Fertilizer, and Livestock Drugs Regulatory Services,
California Department of Food and Agriculture
- Amrith Gunasekara, Ph.D.; Science Advisor to the Secretary; California Department of Food and Agriculture
Last Update: July, 2015
Additional Information:
Links:
- University of California – Fruit & Nut Research and Information Center
- University of California - Nutrient Management for Vegetable, Fruit & Nut Crops
- University of California - Nutrition and Fertilization for Fresh Market Plum, Peach and Nectarine
- University of California - Integrated Pest Management online for Prune and Plum
- Dried Plum Research Reports Database
- California Dried Plum Board
References:
Nitrogen
- Beutel, J., Uriu, K., Lilleland, O., 1976. Leaf analysis for California deciduous fruits. In: Reisenauer, H.M. (Ed.) Soil and Plant-Tissue Testing in California. University of California Cooperative Extension Bulletin 1879. pp. 15-17.
- Bondada, B.R., Syvertsen, J.P., Albrigo, L.G., 2001. Urea nitrogen uptake by citrus leaves. HortScience 36, 1061-1065.
- Brown, P.H., Muhammad, S., Saa Silva, S., 2012a. Update 2012: Fertigation trials in almond.
- Brown, P.H., Saa Silva, S., Muhammad, S., 2012b.Development of leaf sampling interpretation methods for almond and development of a nutrient budget approach to fertilizer management in almond. Almond Board of California Final Research Report.
- Brown, P.H. Niederholzer, F., Sepulveda R., 2014. Development of nutrient management tools for prunes. Report submitted to the California Dried Plum Board.
- Buchner, R.P., 2013. Nitrogen management for French prune. Sacramento Valley Regional Prune Notes, February 2013.
- Day, K.R., Johnson, R.S., Crisosto, C., DeJong, T., Klassen, K., Banuelos, G., 2002. Plum nutrition studies. California Tree Fruit Agreement Research Report.
- Day, K.R., Buchner, R.P., 2012. Growing prunes for the fresh market. In: Buchner, R.P. (Ed.). Prune Production Manual. University of California, Division of Agriculture and Natural Resources, Publication 3507. pp. 279-282.
- Doll, D., 2011. Fertilizing young almond trees – a few tips.
- Fulton, A.E., Sanden, B.L., 2012. Managing salinity in soils and water. In: Buchner, R.P. (Ed.). Prune Production Manual. University of California, Division of Agriculture and Natural Resources, Publication 3507. pp. 43-62.
- Holtz, B., 2010. Fertilizing one-year old trees – Be careful!
- Johnson, R.S., Uriu, K., 1989. Mineral nutrition. In: LaRue, J.H., Johnson, R.S. (Eds.). Peaches, Plums and Nectarines: Growing and Handling for the Fresh Market. University of California, Division of Agriculture and Natural Resources, Publication 3331. pp. 68-81.
- Krueger, W., 2006. Summer nutrition and leaf sampling. Yolo-Solano Counties Fruit and Nut Notes, May 2006.
- Kreuger, W., 2008. Considerations for in-season nutrition of prunes. Sacramento Valley Prune News, March 2008.
- LaRue,J.H., 1989. Establishing the orchard. In: LaRue, J.H., Johnson, R.S. (Eds.). Peaches, Plums and Nectarines: Growing and Handling for the Fresh Market. University of California, Division of Agriculture and Natural Resources, Publication 3331. pp. 21-30.
- LaRue, J.H., Gerdts, M., 1973. Growing plums in California. California Experiment Station Extension Service, Circular 563.
- Leece, D.R., Dirou, J.F., 1977. Organosilicone and alginate adjuvants evaluated in urea sprays foliar-applied to prune trees. Communications in Soil Science and Plant Analysis 8, 169-176.
- Leece, D.R., Dirou, J.F., 1979. Comparison of urea foliar sprays containing hydrocarbon or silicone surfactants with soil-appled nitrogen in maintaining the leaf nitrogen concentration of prune trees. Journal of the American Society for Horticultural Science 105, 644-648.
- Liu, G., Williamson, J., 2013. What is urea-triazone nitrogen? University of Florida IFAS Extension Publication HS1233
- Niederholzer, F.J.A., 2007. Improving the nutrient efficiency of tree crops. California Plant and Soil Conference 2007, February 6&7, 2007, Sacramento.
- Niederholzer, F.J.A., 2011. Using urea efficiently. Glenn County Orchard Facts, April, 2011.
- Niederholzer, F., 2012. Nitrogen use efficiency in almonds. Sacramento Valley Almond News, April 2012.
- Niederholzer, F.J.A., 2013. Efficient nitrogen management in prune production. Sacramento Valley Regional Prune Newsletter, April, 2013.
- Niederholzer, F.J.A., 2014. Prune orchard fertility review. Glenn County Orchard Facts, January 2014.
- Niederholzer, F.J.A., Buchner, R.P., Southwick, S.M., 2012. Nutrition and fertilization. In: Buchner, R.P. (Ed.). Prune Production Manual. University of California, Division of Agriculture and Natural Resources, Publication 3507. pp. 151- 168.
- Olson, W., Andris, H., Buchner, R., Holtz, B., Klonsky, K., Krueger, W., Walton, J., 2004. Integrated prune farming practices (I.P.F.P.)- 2004. A six year summary. Report submitted to the California Dried Plum Board.
- Olson, W., Ramos, D., Yeager, J., Uriu, K., Pearson, J., 1982. Efficient nitrogen application timing in prune production. Report submitted to the California Dried Plum Board.
- Olson, W., Uriu, K., Sibbett, S., Krueger, W., Weakley, C., 1986. Verification of field nitrogen timing trial results- 1986 prune nitrogen survey. Report submitted to the California Dried Plum Board.
- Sibbett, G.S., Southwick, S., 1993. Comparison of foliar vs soil applied nitrogen for maintaining its sufficiency in French prune. Report submitted to the California Dried Plum Board.
- Sibbett, G.S., Southwick, S., Yeager, J., 1991. Effect of foliar applied urea on percent leaf N and fruit size of French prune. Report submitted to the California Dried Plum Board.
- Southwick, S.M., Rupert, M.E., Yeager, J.T., Weis, K.G., DeJong, T., Shackel, K., Bonin, A., 1996. Nitrogen fertigation of young prune trees and effects on horticultural performance: 1996 Final report. Report submitted to the California Dried Plum Board.
- Southwick, S.M., Rupert, M.E., Yeager, J.T., Lampinen, B.D., DeJong, T.M., Weis, K.G., 1999. Effects of nitrogen fertigation on fruit yield and quality of young 'French' prune trees. Journal of Horticultural Science & Biotechnology 74, 187-195.
- Uriu, K., 1981. Soil and plant analysis and symptomology for diagnosis of mineral deficiencies and toxicities. In: Ramos, D.E., (Ed.). Prune Orchard Management. University of California Division of Agricultural Sciences Special Publication 3269. pp. 89-97.
- Weinbaum, S.A., 1978. Feasibility of satisfying total nitrogen requirement of non-bearing prune trees with foliar nitrate. Hortscience 13, 52-53.
- Weinbaum, S.A., Merwin, M.L., Muraoka, T.T., 1978. Seasonal variation in nitrate uptake efficiency and distribution of absorbed nitrogen in non-bearing prune trees. Journal of the American Society for Horticultural Science 103, 516-519.
- Weinbaum, S.A., Brown, P.H., Johnson, R.S., 2002. Application of selected macronutrients (N,K)in deciduous orchards: physiological and agrotechnical perspectives. Acta Horticulturae 594, 59-64.
- Weinbaum, S.A., Niederholzer, F.J.A., Ponchner, S., Rosecrance, R.C., Carlson, R.M., Whittlesey, A.C., Muraoka, T.T., 1994. Nutrient uptake by cropping and defruited field-grown 'French' prune trees. Journal of the American Society of Horticultural Science 119, 925-930.
- Zasoski, R.J., 1994. Nitrogen efficiency in drip irrigated almonds.FREP Final Report.
TOP OF PAGE
Phosphorus
- Beutel, J., Uriu, K., Lilleland, O., 1976. Leaf analysis for California deciduous fruits. In: Reisenauer, H.M. (Ed.) Soil and Plant-Tissue Testing in California. University of California Cooperative Extension Bulletin 1879. pp. 15-17.
- Brown, P.H. Niederholzer, F., Sepulveda R., 2014. Development of nutrient management tools for prunes. Report submitted to the California Dried Plum Board.
- California Plant Health Association, 2002. Western Fertilizer Handbook 9th edition. Interstate Publishers, Inc.
- Doll, D., 2011. Fertilizing young almond trees – a few tips.
- Johnson, R.S., Uriu, K., 1989. Mineral nutrition. In: LaRue, J.H., Johnson, R.S.(Eds.). Peaches, Plums and Nectarines: Growing and Handling for the Fresh Market. University of California, Division of Agriculture and Natural Resources, Publication 3331. pp. 68-81.
- Krueger, W., 2006. Summer nutrition and leaf sampling. Yolo-Solano Counties Fruit and Nut Notes, May 2006.
- Fulton, A., 2010. Understanding and applying information from a soil test: Part 2 – NPK.
- Fulton, A.E., Sanden, B.L., 2012. Managing salinity in soils and water. In: Buchner, R.P. (Ed.). Prune Production Manual. University of California, Division of Agriculture and Natural Resources, Publication 3507. pp. 43-62.
- Niederholzer, F.J.A., Buchner, R.P., Southwick, S.M., 2012. Nutrition and fertilization. In: Buchner, R.P. (Ed.). Prune Production Manual. University of California, Division of Agriculture and Natural Resources, Publication 3507. pp. 151-168.
- Olson, W., Andris, H., Buchner, R., Holtz, B., Klonsky, K., Krueger, W., Walton, J., 2004. Integrated prune farming practices (I.P.F.P.)- 2004. A six year summary. Report submitted to the California Dried Plum Board.
- Shear, C.B., Faust, M., 1980. Nutritional ranges in deciduous tree fruits and nuts. In: Janick, J. (Ed.). Horticultural Reviews. The AVI Publishing Company, Inc.
- Southwick, S.M., Rupert, M.E., Yeager, J.T., Weis, K.G., DeJong, T., Shackel, K., Bonin, A., 1996. Nitrogen fertigation of young prune trees and effects on horticultural performance: 1996 Final report. Report submitted to the California Dried Plum Board.
- Strand, L.L., 1999. Integrated Pest Management for Stone Fruits. University of California, Division of Agriculture and Natural Resources, Publication 3389.
- Uriu, K., 1981. Soil and plant analysis and symptomology for diagnosis of mineral deficiencies and toxicities. In: Ramos, D.E., (Ed.). Prune Orchard Management. University of California Division of Agricultural Sciences Special Publication 3269. pp. 89-97.
- Vercambre, G., Pagès, L., Doussan, C., Habib, R., 2003. Architectural analysis and synthesis of plum tree root system in an orchard using a quantitative modelling approach. Plant and Soil 251, 1-11.
Potassium
- Beutel, J., Uriu, K., Lilleland, O., 1976. Leaf analysis for California deciduous fruits. In: Reisenauer, H.M. (Ed.) Soil and Plant-Tissue Testing in California. University of California Cooperative Extension Bulletin 1879. pp. 15-17.
- Brown, P.H. Niederholzer, F., Sepulveda R., 2014. Development of nutrient management tools for prunes. Report submitted to the California Dried Plum Board.
- Buchner, R.P., Olson, W.H., Niederholzer, F.J.A., Reil, W.O., Krueger, W.H., Fulton, A.E., 2012. Prune production calendar of operations in the Sacramento Valley. In: Buchner, R.P. (Ed.). Prune Production Manual. University of California, Division of Agriculture and Natural Resources, Publication 3507. pp. 297.
- Carlson, R.M., Buchanan, J.R., Kapustka, T.E., Uriu, K., 1974. Displacement of fertilizer potassium in soil columns with gypsum. Journal of the American Society for Horticultural Science 99, 221-222.
- Day, K.R., Buchner, R.P., 2012. Growing prunes for the fresh market. In: Buchner, R.P. (Ed.). Prune Production Manual. University of California, Division of Agriculture and Natural Resources, Publication 3507. pp. 279-282.
- DeBuse, C., 2012. Prune orchard nutrition. Sutter-Yuba Counties Pomology Notes, November 2012.
- DeBuse, C., Niederholzer, F.J.A., 2011. In-season potassium fertilization options for prunes. Glenn County Orchard Facts, April, 2011.
- DeVay, J.E., Gerdts, M., English, H., Lukezic., F.L., 1974. Controlling cytospora canker in President plum orchards of California. California Agriculture 28, 12-14.
- Doll, D., 2011. Fertilizing young almond trees – a few tips.
- Doll, D., 2014. Almond orchard nitrogen and potassium fertilization. Presented at the South San Joaquin Valley Almond Symposium, 5/29/2014.
- Fulton, A., 2010. Understanding and applying information from a soil test: Part 2 – NPK.
- Fulton, A.E., Sanden, B.L., 2012. Managing salinity in soils and water. In: Buchner, R.P. (Ed.). Prune Production Manual. University of California, Division of Agriculture and Natural Resources, Publication 3507. pp. 43-62.
- Ghanem, M., Ben Mimoun, M., 2010. Effect of potassium foliar spray on two plum trees cultivars: 'Strival' and 'Black Star'. Acta Horticulturae 874, 83-90.
- Hoagland, D.R., Chandler, W.H., 1932. Some effects of deficiencies of phosphate and potassium on the growth and composition of fruit trees, under controlled conditions. Proceedings of the American Horticultural Society 29, 267-271.
- Johnson, R.S., Uriu, K., 1989. Mineral nutrition. In: LaRue, J.H., Johnson, R.S. (Eds.). Peaches, Plums and Nectarines: Growing and Handling for the Fresh Market. University of California, Division of Agriculture and Natural Resources, Publication 3331. pp. 68-81.
- Krueger, W., 2006. Summer nutrition and leaf sampling. Yolo-Solano Counties Fruit and Nut Notes, May 2006.
- Krueger, W. 2009. Steps for producing large prunes. Western Farm Press, May 2009.
- LaRue, J.H., Gerdts, M., 1973. Growing plums in California. California Experiment Station Extension Service, Circular 563.
- Lilleland, O., 1932. Experiments in K and P deficiencies with fruit trees in the field. Proceedings of the American Society for Horticultural Science 29, 272-276.
- Niederholzer, F.J.A., 2014. Prune orchard fertility review. Glenn County Orchard Facts, January 2014.
- Niederholzer, F.J.A., Buchner, R.P., Southwick, S.M., 2012. Nutrition and fertilization. In: Buchner, R.P. (Ed.). Prune Production Manual. University of California, Division of Agriculture and Natural Resources, Publication 3507. pp. 151-168.
- Olson, W.H, Uriu., K., Carlson, R.M., Krueger, W.H., Pearson,J., 1987. Correcting potassium deficiency in prune trees is profitable. California Agriculture 41, 20-21.
- Olson, W., Andris, H., Buchner, R., Holtz, B., Klonsky, K., Krueger, W., Walton, J., 2004. Integrated prune farming practices (I.P.F.P.)- 2004. A six year summary. Report submitted to the California Dried Plum Board.
- Pope, K., 2014. Potassium nutrition- maintaining optimum levels by replacing what you've lost. Glenn County Orchard Facts, October 2014.
- Shear, C.B., Faust, M., 1980. Nutritional ranges in deciduous tree fruits and nuts. In: Janick, J. (Ed.). Horticultural Reviews. The AVI Publishing Company, Inc.
- Strand, L.L., 1999. Integrated Pest Management for Stone Fruits. University of California, Division of Agriculture and Natural Resources, Publication 3389.
- Southwick, S.M., Olson, W., Yeager J., Weis, K.G., 1996a. Optimum timing of potassium nitrate spray applications to 'French' prune trees. Journal of the American Society for Horticultural Science 121, 326-333.
- Southwick, S.M., Rupert, M.E., Yeager, J.T., Weis, K.G., DeJong, T., Shackel, K., Bonin, A., 1996b. Nitrogen fertigation of young prune trees and effects on horticultural performance: 1996 Final report. Report submitted to the California Dried Plum Board.
- Uriu, K., Carlson, R.M., Henderson, D.W., Shulbach, H., Aldrich, T.M., 1980. Potassium fertilization of prune trees under drip irrigation. Journal of the American Society for Horticultural Science105, 508-510.
- Uriu, K., 1981. Soil and plant analysis and symptomology for diagnosis of mineral deficiencies and toxicities. In: Ramos, D.E., (Ed.). Prune Orchard Management. University of California Division of Agricultural Sciences Special Publication 3269. pp. 89-97.
- Vercambre, G., Pagès, L., Doussan, C., Habib, R., 2003. Architectural analysis and synthesis of plum tree root system in an orchard using a quantitative modelling approach. Plant and Soil 251, 1-11.
- Weinbaum, S.A., Niederholzer, F.J.A., Ponchner, S., Rosecrance, R.C., Carlson, R.M., Whittlesey, A.C., Muraoka, T.T., 1994. Nutrient uptake by cropping and defruited field-grown 'French' prune trees. Journal of the American Society for Horticultural Science 119, 925-930.
TOP OF PAGE
Zinc
- California Plant Health Association, 2002. Western Fertilizer Handbook 9th Ed. Interstate Publishers, Inc.
- Connell, J., 2013. Prune tissue sampling for leaf analysis. Sacramento Valley Regional Prune Newsletter, April, 2013.
- DeBuse, C., 2012. Prune orchard nutrition. Sutter-Yuba Counties Pomology Notes, November 2012.
- Johnson, R.S., Crisosto, C., Andris, H., Day, K., Holtz, B., Beede, B., Klassen, K., 2003. The effect of nutrient deficiencies on stone fruit production and quality. FREP Final Report.
- Johnson, R.S., Day, K.R., 2013. Zinc Deficiency and Correction in California Plum Orchards. Acta Horticulturae 985, 189-192.
- Krueger, W., 2006. Summer nutrition and leaf sampling. Yolo-Solano Counties Fruit and Nut Notes, May 2006.
- LaRue, J.H., Gerdts, M., 1973. Growing plums in California. California Experiment Station Extension Service, Circular 563.
- Niederholzer, F.J.A., Buchner, R.P., Southwick, S.M., 2012. Nutrition and fertilization. In: Buchner, R.P. (Ed.). Prune Production Manual. University of California, Division of Agriculture and Natural Resources, Publication 3507. pp. 151-168.
- Niederholzer, F.J.A., 2014. Prune orchard fertility review. Glenn County Orchard Facts, January 2014.
- Olson, W., Andris, H., Buchner, R., Holtz, B., Klonsky, K., Krueger, W., Walton, J., 2004. Integrated prune farming practices (I.P.F.P.)- 2004. A six year summary. Report submitted to the California Dried Plum Board.
- Strand, L.L., 1999. Integrated Pest Management for Stone Fruits. University of California, Division of Agriculture and Natural Resources, Publication 3389.
- Swietlik,D., 2002. Zinc Nutrition of Fruit Crops. Horttechnology 12, 45-50.
- Uriu, K., 1981. Soil and plant analysis and symptomology for diagnosis of mineral deficiencies and toxicities. In: Ramos, D.E., (Ed.). Prune Orchard Management. University of California Division of Agricultural Sciences Special Publication 3269. pp. 89-97.
